Protocol: Drug screening of cultivated cells
Use VersaLive to screen up to five drugs at the same time
Materials
- VersaLive Starter Kit
- VersaLive chips
- Cell suspension (10 μL/chip or more, 1—5•106 cells/mL)
- Cell medium
- 1X PBS
- Mineral oil for cell culture
- Stereomicroscope
Channel wetting
The wetting of the channels is carried out within minutes after the bonding procedure.
- Add 10 μL of PBS in port B of the chip and observe at the stereomicroscope the wetting of all channels up to the remaining ports.
- If capillary action is not enough to achieve the wetting of all channels:
- Fill all ports with 10 μL of PBS
- Place the chip in a desiccator
- Degas the chip applying vacuum for 15 minutes
- Check the result at the stereomicroscope and repeat if necessary
- VersaLive chips can be sealed with adhesive tape to prevent evaporation and stored at 4˚C for at least one week. Do not use mineral oil before cell loading
This is a good pause point
Cell loading and static cell culture
- Empty all ports from the PBS used for the channel wetting
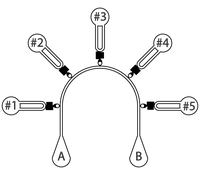
- Add 10 μL of cell suspension in port B. Cells will start flowing through the main channel and entering the chambers
- When a given chamber is filled with enough cells, add 10 μL of cell medium to the respective port to stop its filling
- When all chambers are filled, fill port A with 20 μL of cell medium
- Remove the cell suspension from port B and rinse it with cell medium or PBS
- Add 20 μL of cell medium to port B
- Fill up to 20 μL all chamber ports #1 to #5
- Add 2.5 μL of mineral oil to each reservoir to prevent the evaporation of the cell medium
- Place the chip in the incubator at 37˚C and 5% of CO2 until the cells are adherent to the bottom of the chip (a few hours or days, depending on the cell line)
Perfusion for drug screening
- Remove content from ports A and B
- Replace content from ports #1 to #5 with different drugs
- Add 2.5 μL of mineral oil in input ports #1 to #5